Technology advances have made cancer therapies promising for treating newly diagnosed patients with localized tumors. However, recurrent and metastatic tumors often show limited responses, highlighting the urgent need to address the therapeutic gap.
Tumor recurrence and metastasis are spatiotemporally complicated, progressing over years and involving multiple organs. To develop innovative therapeutic strategies against tumor recurrence and metastasis, we aim to advance technologies that unravel the underlying spatiotemporal mechanisms at various levels of complexity.

- At the human body level, we apply spatiotemporal omics and cancer pathology to reveal the mechanisms that drive tumor recurrence and metastasis.
- At the tissue level, we engineer biomimetic tumor models to recapitulate the dynamic tumor microenvironment for studying the causes and effects during tumor recurrence and metastasis.
- At the molecular level, we advance our tumor models to high-throughput drug screening platforms to discover promising therapeutics while minimizing the reliance on animal models prior to in vivo testing.

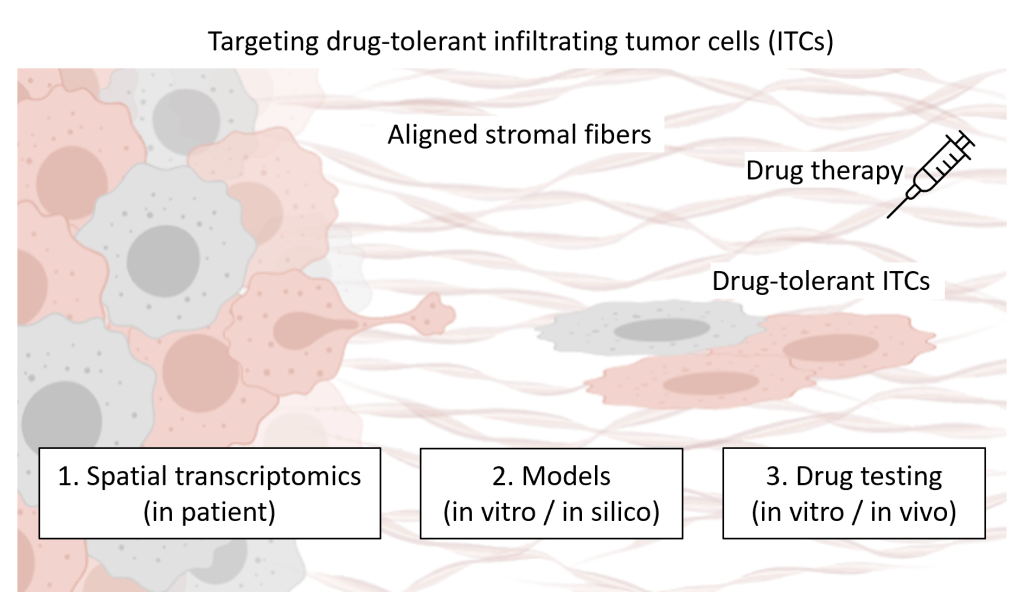
Our lab is currently focused on infiltrating tumor cells (ITCs) at the invasion front on aligned tumor stromal fibers. ITCs, which separate from the primary tumor mass and invade the surrounding stroma, represent the initial step toward metastasis. Tumor stroma, characterized by aligned extracellular matrix fibers remodeled by ITCs, is a consistent feature observed in cancer patients. The aligned fibers, in turn, act as stromal highways, facilitating persistent cancer cell invasion.
It may be argued that most cancers are diagnosed after cancer cells have already disseminated to distant sites, forming micro- or macro-metastases. Additionally, surgical excision often removes ITCs at the invasion front along with the primary tumor. Despite these concerns, growing evidence suggests that ITCs at the invasion front closely mirror metastatic cells, with their plasticity enabling adaptation to dynamic microenvironments. Furthermore, our previous study demonstrated that aligned stromal topography induces drug resistance in ITCs, increasing the risk of tumor recurrence. Therefore, studying ITCs offers a unique window to develop treatments specific for tumor recurrence and metastasis.

Spatiotemporal Tumor Biology
Spatiotemporal Molecular Profiling and Drug Screening of Drug Tolerant Infiltrative Residual Breast Cancer to Identify Therapeutics Targeting Tumor Recurrence. PI: Su CY. 2030 Cross-Generation Young Scholars Program, Emerging Young Scholars, NSTC, Taiwan. 2023/09 – now (4-year grant)
- In this ongoing project, we integrate cutting-edge technologies, including spatial transcriptomics analysis of human cancer samples, in vitro 3D infiltrating tumor cell models, in silico computational simulation, high-throughput drug screening, and in vivo drug testing, to address the question, “How to prevent breast cancer recurrence by targeting drug-tolerant infiltrating tumor cells post-drug therapy?"
Tumor stromal topography promotes chemoresistance in migrating breast cancer cell clusters. Su CY, Wu A, Dong Z, Miller CP, Suarez A, Ewald AJ, Ahn EH, Kim DH. Biomaterials. 2023
- We developed a hybrid nanopatterned model to study breast cancer cell clusters at the migration front with aligned stromal topography. Our findings revealed that topography induces drug resistance of breast cancer cell clusters to chemotherapy drugs by activating the AhR/CYP1 pathways. Inhibiting the AhR/CYP1 pathway restores reactive oxygen species-mediated drug sensitivity to migrating cancer cell clusters, suggesting a potential therapeutic direction for preventing metastatic recurrence.

Matrix Anisotropy Promotes a Transition of Collective to Disseminated Cell Migration via a Collective Vortex Motion. Su CY, Matsubara T, Wu A, Ahn EH, Kim DH. Advanced Biology. 2023
- We demonstrated that matrix anisotropy drives a transition from collective to disseminated cell migration through a unique vortex-like collective motion. Using biomimetic tumor models and quantitative imaging, we uncovered a microenvironmental mechanism that promotes cancer dissemination.

Tumor Model Engineering
Engineering a 3D collective cancer invasion model with control over collagen fiber alignment. Su CY, Burchett A, Dunworth M, Choi JS, Ewald AJ, Ahn EH, Kim DH. Biomaterials. 2021. International Patent. US Patent.
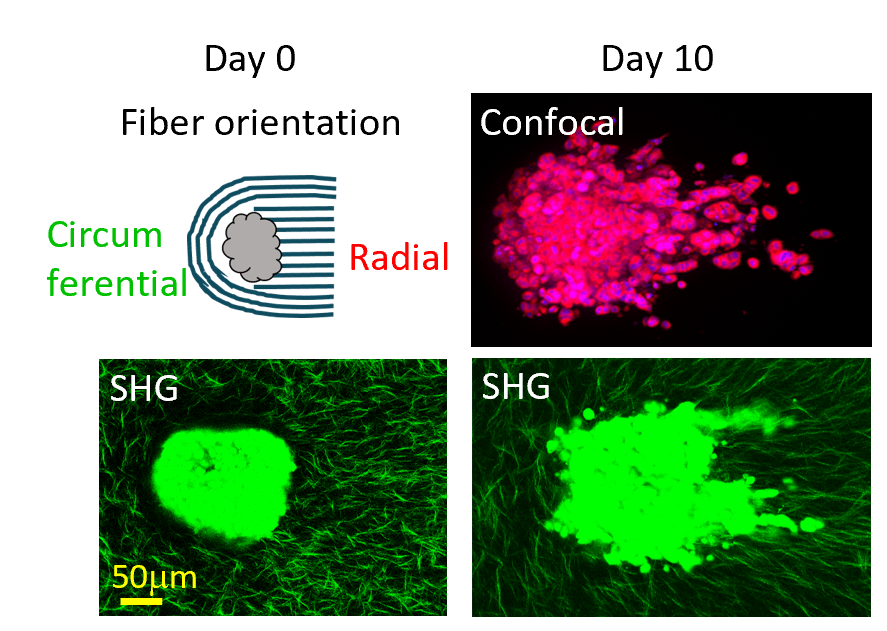
- We developed a patented phase-specific, force-guided method to create a 3D dual-topographical tumor model featuring a large collagen gel embedded with over 1,000 tumor spheroids or organoids. Each tumor spheroid/organoid is surrounded by radially aligned collagen I fibers on one side and circumferentially oriented fibers on the other. The model enables the distinction of invasion patterns and capacities of tumor spheroids and organoids. We anticipate that the 3D dual topographical model will have broad utility for studying collective tumor invasion and holds promise for identifying therapeutic agents targeting cancer invasion.
Complete list of published works on Google Scholar, Scopus, ORCID
Teaching
Cancer Biology and Tumor Model Engineering course on NYCU OCW.

